Estrous Cycle of the Mare Evaluated by Fecal Progesterone, Estrone Conjugates and Immunoreactive Pregnanediol-Glucuronide-Like Metabolites
Brandon Carpenter, B.S., Vicki Barkhuff, B.S., and J.F. Kirkpatrick, Ph.D.
1992
Summary
The estrous cycles of four domestic mares were characterized by means of urinary estrone conjugates (E1C) and immunoreactive pregnanediol-like progesterone metabolites (iPdG). The urinary E1C concentrations were compared to fecal E1C concentrations, and urinary iPdG concentrations were compared to fecal iPdG and progesterone (Po) concentrations, in order to evaluate the use of fecal steroids for assessment of ovarian function. Correlation coefficients for urinary steroid conjugates versus fecal steroid conjugates or progesterone ranged from r = 0 to r = 0.83, but fecal E1C, iPdG, and Po all paralleled the qualitative changes in ovarian endocrine function assessed by urinary steroid conjugate analysis. Fecal progesterone provided a more accurate assessment of the luteal phase than fecal iPdG. The results indicate that ovarian endocrine activity in the mare can be evaluated through the use of fecal steroids or their metabolites, and that this approach may be useful for the study of reproduction in free-roaming feral horses.
Introduction
Characterizing endocrine events associated with reproduction in large free-roaming animals can be a difficult and dangerous task, due to the necessity of obtaining blood samples. Until recently it was necessary to capture and restrain or tranquilize wild species in order to collect blood. An alternative approach to the study of reproductive endocrine events in free-roaming wildlife is based on the measurement of urinary and fecal steroids or their metabolites. The technology which permits remote monitoring of reproductive status of captive exotic mammals through urinary steroid metabolites (Loskutoff et al. 1983; Lasley 1985; Lasley and Kirkpatrick 1991). This same technology was next extended to pregnancy detection in free-roaming feral horses (Kirkpatrick et al. 1988, 1990a,b). In this case, a single urine sample, collected from the ground, and the measurement of a pregnancy-specific hormone metabolite was all that was necessary. The ovarian endocrine changes associated with the estrous cycle are dynamic and require frequent urine sampling in order to visualize the sequential events of ovarian function in free-ranging wildlife (Lasley and Kirkpatrick 1991; Monfort et al. 1991)
The collection of urine from free-roaming wildlife can be time-consuming and in some cases dangerous. The use of fecal steroid evaluation has proven successful with a number of domestic and captive exotic species (Schwarzenberger et al. 1990; Bamberg and Schwarzenberger 1990). Pregnancy diagnosis has been accomplished by the measurement of fecal steroids or their metabolites in feral horses (Kirkpatrick et al. 1990a, 1991a; Lucas et al. 1990) and caribou (Rangifer tarandus) (Messier et al. 1990).
It is also possible to evaluate cyclic ovarian endocrine activity in wildlife by means of fecal steroids or their metabolites. Desaulniers et al. (1989) monitored the luteal phase of the domestic cow estrous cycle by means of fecal progesterone. Our study was conducted to evaluate the estrous cycle of the domestic mare by means of fecal progesterone (Po), estrone conuugates (E1C) and immunoreactive pregnanediol-glucuronide-like progesterone metabolites (iPdG) and to compare the accuracy of fecal steroid analysis with the results of urinary steroid analysis.
Materials and Methods
Four sexually mature, domestic, non-breeding mares (Equus caballus) were used for this study. The mares were between 6 and 20 years old and were pastured on approximately 8 hectares of grass, with free-standing water available ad libitum. Matched urine and fecal samples were collected from each mare every other day from April 23 to July 1. The urine samples were removed from newly saturated soil immediately after urination as described by Kirkpatrick et al. (1988). Fecal samples were collected and stored in plastic bags. Both urine and fecal samples were placed on ice at the time of collection and frozen until assay.
Urine samples were diluted 1:100 in distilled water (dH20) for the measurement of E1C and 1:1 for the measurement of iPdG. The E1C enzyme immunoassay has been previously described by Munro et al. (1991). The intra- and interassay coefficients of variation were 5.8% (n = 4) and 14.2% (n = 8). In order to account for differences in urine concentrations, the urine samples were diluted 1:100 with dH2O and analyzed for creatinine (Cr) by the microcolorimetric method of Taussky (1954), and E1C and iPdG were reported as ng/mg Cr. Urinary E1C and iPdG were plotted for the duration of the study period and permitted assessment of 2 complete estrous cycles for each mare. The best defined estrous cycle for each mare was used for comparison with fecal hormone concentrations.
Wet fecal samples were weighed and approximately 3 g were mixed with 7.0 ml of dH2O, shaken for several hours and incubated at 10 o C overnight. This slurry was centrifuged at 2,500 rpm for 15 minutes and the supernatant was recovered for measurement of E1C and iPdG. Following centrifugation, the fecal pellet was dried under a continuous stream of air at 40 o C. The dried material was ground fine in a mortar and pestle and 0.25 g was rehydrated with 0.5 ml dH2O and extracted 3 times with 3 ml of diethyl ether each time, as described by Desaulnier et al. (1989). The ether extracts were dried under air, the residue redissolved in 1.0 ml 95% ethyl alcohol and stored at 5 o C until assay. The ethanol was diluted 1:100 and 20 µl were assayed for Po by enzyme immunoassay as described my Munro and Stabenfeldt (1984). Progesterone and concentrations were reported as ng/g dry feces. The intra- and interassay coefficients of variation for the Po EIA were 9.6% (n = 4) and 16.3% (n = 4). Fecal Po and iPdG were compared to urinary E1C concentrations for each estrous cycle.
Results
Urinary E1C and iPdG concentrations during the 69-day collection period revealed a pattern consistent with ovulation and non-conceptive luteal phase Po elevation which is consistent with the estrous cycle of the mare (Daels et al. 1991; Kirkpatrick et al. 1990c). Each estrous cycle consisted of a preovulatory E1C peak, a concomitant iPdG nadir which is required for ovulation, and a subsequent elevation of iPdG reflecting the luteal phase of the cycle. Among the four mares, eight complete estrous cycles were identified.
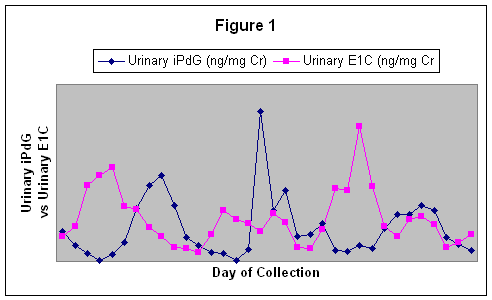
Figure 1.
Three consecutive estrous cycles based on Urinary E1C and iPdG for the mare named Granny.
The two line graph plots indicate a correlation between iPdG an E1C pre-ovulatory estrogen peaks, approximate time of ovulation, and the luteal phase progesterone peaks.
Three consecutive cycles, based on urinary E1C and iPdG, for the mare named Granny are shown in Figure 1. Fecal E1C concentrations reflected the same patterns as that seen with the urinary E1C and the correlation coefficients between urinary E1C and fecal E1C ranged from r = 0.01 to r = 0.63. An estrous cycle characterized by urinary and fecal E1C is shown in figure 2A.
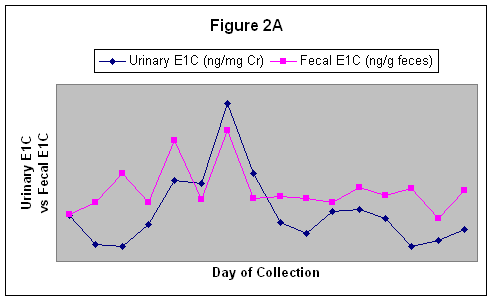
Figure 2A. A single estrous cycle
A clear correlation between Urinary iPdG and Fecal E1C.
Fecal iPdG and Po also reflected the urinary iPdG patterns over the course of the estrous cycles. The correlation coefficients for urinary iPdG and fecal iPdG ranged from r = 0.08 to r = 0.42. An estrous cycle reflecting urinary and fecal iPdG is shown in figure 2B. The correlation coefficients for urinary iPdG and fecal Po ranged from r = 0.44 to r = 0.83. An estrous cycle reflecting urinary iPdG and fecal Po is shown in figure 2C.
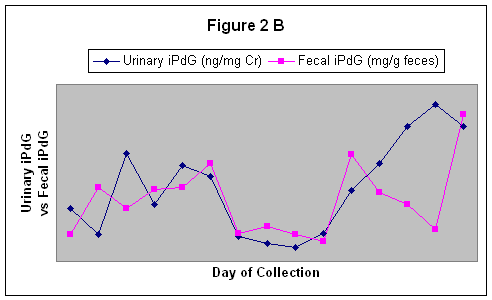
Figure 2 B.
A correlation between Urinary iPdG and Fecal iPdG.
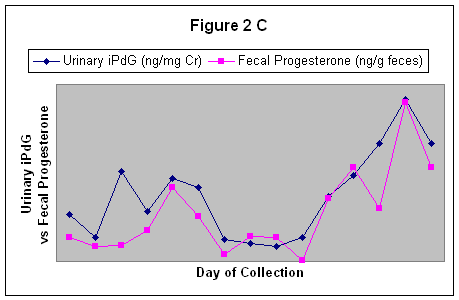
Figure 2 C. A correlation between Urinary iPdG and Fecal Progesterone.
Discussion
The remote monitoring of ovarian function in the mare is possible by means of fecal E1C, iPdG and Po. There is a large quantitative difference between urinary E1C and iPdG values and fecal E1C and iPdG values, but the fecal values accurately reflect the qualitative changes occurring in urinary E1C and iPdG during the estrous cycle. The quantitative differences are explained by the fact that the majority of estrogen and Po metabolites in the mare are excreted in the urine rather than the feces. Estrogens in the vascular space are metabolized in the liver and conjugated to either sulfate or glucuronic acid (Daels et al. 1991). From the liver, these conjugates pass into the gastrointestinal tract where the majority are resorbed back into the blood, transported to the kidney and excreted in the urine. However, a smaller portion of the E1C is trapped in the gastrointestinal tract and excreted in the feces (Kirkpatrick et al. 1991a).
The precise nature of the iPdG in the mare is not known, but consists of three progesterone metabolites, all of which are more polar than pregnanediol-3-glucuronide (PdG) and which are highly correlated with plasma progesterone (Kirkpatrick et al. 1990c). It is probable that the appearance of iPdG in the feces occurs in the same manner as fecal E1C. Fecal Po concentrations in the mare are considerable lower than those found in the cow (Desaulniers et al. 1989), and may reflect differenced in Po metabolism between the two species. The primary Po metabolite in the domestic cow (Bos taurus) (Feher 1975) and the North American bison (Bison bison) (Kirkpatrick et al. 1991b) is PdG, however, PdG is absent in the mare (Kirkpatrick et al. 1990c.) Conversely, iPdG, the primary Po metabolite in the mare, is absent in the cow and bison.
The correlation coefficients between urinary steroid metabolites and fecal metabolites or progesterone were the lowest for iPdG, and the strongest for progesterone. This suggests that fecal progesterone is a more accurate measure of luteal function in mares than iPdG. Despite some very low correlation coefficients, particularly for urinary vs. fecal E1C and iPdG, all 3 fecal hormones provided a qualitative view of ovarian endocrine activity that was useful for identifying the time of estrus, the occurrence of ovulation, and the presence of a luteal phase in the mare. This provides the field biologist with a relatively simple approach to monitoring ovarian function among feral mares, without the physiological stresses of capture and the costs of immobilization. The same basic techniques should be applicable to a variety of free-roaming exotic ungulates, although care must be taken to identify the specific fecal steroids or their metabolites and to validate their accuracy in identifying reproductive events in each species.
References
1. Loskutoff, N.M., Ott,, J.E., Lasley, B.L., Strategies for assessing ovarian function in exotic species. J. Soo Anim. Med. 14:3-12, 1983.
2. Lasley, B.L., Methods for evaluating reproductive function in exotic species. Adv. Vet. Sci. Comp. Med 30:209-228, 1985.
3. Lasley, B.L., Kirkpatrick, J.F., Monitoring ovarian function in captive and free-ranging wildlife by means of urinary and fecal steroids. J. Zoo Wildl. Med. 22:23-31, 1991.
4. Kirkpatrick, J.F., Kasman, L.H., Lasley, B.L., Turner, J.W. Jr., Pregnancy determination in uncaptured feral horses. J. Wildl. Manag. 52:305-308, 1988.
5. Kirkpatrick, J.F., Shideler, S.E., Turner, J.W. Jr., Pregnancy determination in uncaptured feral horses based on steroid metabolites in urine-soaked snow and free steroids in feces. Can. J. Zool. 68:2576-2579, 1990a.
6. Kirkpatrick, J.F., Liu, I.M.K., Turner, J.W. Jr., Remotely delivered immunocontraception in feral horses. Wildl. Soc. Bull. 18:326-330, 1990b.
7. Monfort, S.L., Arthur, N.P., Wildt, D.E., Monitoring ovarian function and pregnancy by evaluating urinary oestrogen conjugates excretion in semi-free-ranging Przewalski’s horses (Equus przewalskii). J. Reprod. Fert. 91:155-164, 1991.
8. Schwarzenberger, F., Mostl, E., Bamberg, E., Monitoring the oestrous cycle in farm animals by measuring gestagens in feces. J. Steroid Biochem. 36:129-135, 1990.
9. Bamberg, E., Schwarzenberger, E., Fecal steroid assay for monitoring estrous cycles and pregnancy. IVth Congr. Int. Soc. Anim. Clin. Biochem., Davis, CA., pp.95-99, 1990.
10. Kirkpatrick, J.F., Shideler, S.E., Lasley, B.L., Turner, J.W. Jr., Pregnancy determination in uncaptured feral horses by means of fecal steroid conjugates. Theriogenology 35:753-759, 1991a.
11. Lucas, Z., Raeside, J.I., Betteridge, K.J., The incidences of pregnancy and pregnancy loss in the feral horses of Sable Island based on field observations and determination of fecal oestrogen concentrations. Proc. Int. Symp. Equine Reprod., Deauville, Ftance, July 1-7, pp.146-147, 1990.
12. Messier, F., Desaulniers, D.M., goff, A.K., Nault, R., Tatenaude, R., Crete, M., Caribou pregnancy diagnosis from immunoreactive progestins and estrogens excreted in feces. J. Wild. Manage. 54:279-283, 1990.
13. Desaulniers, D.M., Goff, A.K., Betteridge, K.J., Rowell, J., Flood, P.F., Reproductive hormone concentrations in feces during the estrous cycle and pregnancy in cattle (Bos Taurus) and muskoxen (Ovibus moschatus). Can. J. Zool. 67:1148-1154, 1989.
14. Munro, C.J., Stabenfeldt, G.H., Overstreet, J.W., Cragun, J.R., Addiego, L.A., Lasley, B.L., Serum and urinary ovarian steroids assessed by radioimmunoassay and enzyme immunoassay. Clin. Chem. 37:838-844, 1991.
15. Shideler, S.E., Tell, L., Owiti, G., Laughlin, L., Chatterton, R., Lasley, B.L., The relationship of serum estradiol and progesterone concentrations to the enzyme immunoassay measurement of urinary estrone conjugates and immunoreactive pregnanediol-3-glucuronide in Macaca mulatta. Am. J. Primatol. 22:113-122, 1990.
16. Kirkpatrick, J.F., Lasley, B.L., Shideler, S.E., Urinary steroid evaluations to monitor ovarian function in exotic ungulates: VII. Urinary progesterone metabolites in the Equidae assessed by immunoassay. Zoo. Biol. 9:341 -348, 1990c.
17. Taussky, H.H., A microcolorimetric determination of creatinine in urine by the Jaffe reaction. J. Biol. Chem. 208:853-861, 1954.
18. Munro, C.J., Stabenfeldt, G.H., Development of a microtiter plate enzyme immunoassay for the determination of pregnancy. J. Endocr. 101:41-49, 1984.
19. Daels, P.F., Ammon, D.C., Stabenfeldt, G.H., Liu, I.M.K., Hughes, J.P., Lasley, B.L., Urinary and plasma estrogen conjugates, estradiol, and estrone concentrations in nonpregnant and early pregnant mares. Theriogenology 35:1001-1015, 1991.
20. Feher, T., Gas chromatographic evidence of pregnanediols in non-pregnant cow urine. Act. Vet. Helvetica 25:241-244, 1975.
21. Kirkpatrick, J.F., Kincy, V., Bancroft, K., Shideler, S.E., Lasley, B.L., The estrous cycle of the North American bison (Bison bison) characterized by urinary pregnanediol-3-glucuronide. J.Reprod. Fert. 93: (in press), 1991b.


